 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:07pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:07pm On Jul 22, 2018 |
Aceed:
A man whose mass is 80kg stands on a
spring weighing machine inside an
elevator. What is the reading of the
weighing machine when the elevator is
coming to rest with a retardation of
4.0ms^-2?
Solution. The apparent weight of the man when the elevator moves up with an acceleration is given by: W = m(g + a) But in this question,the elevator is coming to rest(upwards) with a deceleration, -a . .: the equation becomes: W = m(g - a). Substituting the values, we have that: W = 80 x (10 - 4) = 80 x 6 = 480N or 48 Kg. |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:46pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:46pm On Jul 22, 2018 |
Aceed:
An object of mass 1kg falls a distance
of 5m onto a horizontal surface and
rebounds to a vertical height of 2m.
Calculate the change in momentum.
Solution. Initial velocity of the body before falling from a distance of 5m = 0m/s. Final velocity(V) of the body just before hitting the horizontal surface can be gotten by using the formula: V 2 = u 2 + 2gh = 0 + 2 x 10 x 5m = 100. .: V = squaroot of 100 = 10m/s Final velocity after the body reached its maximum height on rebound is of course 0m/s......and Initial velocity(u) with which the body rebounds to a height of 2m can be gotten by the formula: V 2 = u 2 - 2gh. Note the negative sign here because the body is now moving against gravity on rebounding. Here, V = 0m/s. .: 0 = u 2 - 2gh - u 2 = -2gh .: u = squareroot of (2 x 10 x 2) = 6.325 m/s Change in momentum = mv - mu. If momentum of the object on hitting the floor is positive,it implies that its momentum on moving away from the floor is negative. Where m = 1kg..... .: Change in momentum = mv - (- mu) = mv + mu = (1 x 10) + (1 x 6.325) = 16.325 Ns |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:57pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:57pm On Jul 22, 2018 |
Aceed:
The horizontal door of a submarine at a depth of 500m has an area of 0.4m^2.
Calculate the force exerted by the sea water on the door at this depth.
(R.D of seawater = 1.03, atm = 1.01x 10^5Nm^-2, Density of pure water = 1000kgm^-3, g = 10ms^-2)
Solution: Pressure = Density x g x height Density of seawater = 1030 .: Pressure = 1030 x 10 x 500m = 5150000 Nm 2 . But, Pressure = Force/Area. .: The force exerted by the sea water on the door of area 0.4m 3 at a depth of 500m = Pressure x Area = 5150000 x 0.4 = 2060000N = 2.06 x 106 N |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:32pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:32pm On Jul 22, 2018 |
Aceed:
What is the upthrust on a body which displaces
0.6m^3 of water, 0.5m^3 of methylated spirit?
(density of water = 10^3 kgm^-3, density of
methylated spirit = 0.8 x 10^3 kgm^-3 and
g = 10ms^-2)
Solution: Upthrust = density x volume x acceleration due to gravity(g). For the body that displaced 0.6m 3 of water; Upthrust = 1000 x 0.6 x 10 = 6000N For the body that displaced 0.5m 3 of methylated spirit; Upthrust = 800 x 0.5 x 10 = 4000N |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:13pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:13pm On Jul 22, 2018 |
Aceed:
What is the efficiency of a cell
with internal resistance of 3ohms,
when it supplies current to a 7ohms
resistor?
Solution. The efficiency of a cell can be given by the formula: Efficiency = External Resistance,R/ Internal Resistance,r + External Resistance,R. x 100% i.e Efficiency = (R/r + R) x 100% From the question, External Resistance,R = 7 ohms .....and Internal Resistance,r = 3 ohms Substituting the values in the equation above, we have that: Efficiency = (7/3 + 7) x 100% = (7/10) x 100% = 0.7 x 100% = 70% |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:17pm On Jul 22, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:17pm On Jul 22, 2018 |
The correct answers are marked with * Nltaliban:
[7/21, 20:47] Feranmi: © 2018 POST UTME TOP SECRETS FORUM
*2018 LIKELY POST UTME QUESTIONS*
*CHEMISTRY : 21/07/18(19:00 GMT* )
*PART ONE*
*10 QUESTIONS : 5 MINUTES*
*INSTRUCTIONS*
Ensure that you take this test within the Stipulated time
Ensure that you Submit all yours answers together and at once to the Forum before the Authentic answers are released
Ensure that you Post your Scores to the Forum for My Evaluation.
1. The phenomenon observed when dust particles collide randomly in a beam of sunlight is known as
A. Tyndal effect
B. diffusion
C. osmosis
D. brownian movement *
2. The mass number of an element is the sum of its
A. electrons, neutrons and protons
B. electrons and protons
C. protons and neutrons *
D. orbital electrons
3. Radon is used as a tracer in medical research because it
A. is monatomic
B. is radioactive *
C. is a noble gas
D. has a complete valence
4. What is the most probable group of an element which is a soft, silvery white solid and reacts violently with water?
A. Group 0
B. Group 1 *
C. Group 4
D. Group 6
5. In linear molecules, the bond angle is
A. 90°
B. 140°
C. 109°
D. 180° *
6. An acid is a substance which in the presence of water produces
A. salts
B. oxygen
C. effervescence
D. hydroxonium ions *
7 A sodium atom and a sodium ion have the same
A. number of neutrons *
B. number of electrons
C. electric charge
D. electronic structure
8. If the atomic number of an element X is 11 and that of nitrogen is 7, the most likely formula of the nitride of X is
A. X3N *
B. XN3
C. X3N2
D. N2X
9. An increase in the pressure of a gas results in a decrease in its
A. mass
B. vapour density
C. volume *
D. temperature
10. Zinc Oxide is a
A. Basic Oxide
B. Acidic Oxide
C. Amphoteric Oxide *
D. Neutral Oxide
|
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:06pm On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:06pm On Jul 21, 2018 |
.... |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 6:55pm On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 6:55pm On Jul 21, 2018 |
Eben331:
A resonance tube is 40cm long. The tube is three-quarter full. What is the frequency of the turning fork placed near the mouth of the tube? (velocity of sound in air=334m/s) (A) 2511Hz (B) 1670Hz (C) 835Hz (D) 345Hz PLS SHOW YOUR WORKINGS!! I think BiafranDel is correct..... I got 835Hz which is C |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 2:01pm On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 2:01pm On Jul 21, 2018 |
Eben331:
Tell Damayogenius18 to come to Nairaland, it is not all of us that have access to WhatsApp. You also should visit Nairaland often. Yesooooo.......na here e dey happen. Everybody should come to nairaland abeg. Facebook I know, Nairaland I know,..........Who be Whatsapp  |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:39am On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:39am On Jul 21, 2018 |
esivue007:
@drbest jc,i think it will be right if u post d glycolysis question in d medical thread There is no need.'BiafranDel' is very correct........doctors are here in this thread also   Back to the question... Aceed:
The enzyme of the glycolytic pathway
is located in the?
(a)mitochondria
(b) golgi apparatus
(c) cytoplasm
(d) Nucleus
When I was reading my biology textbook(IDODO UME COLLEGE BIOLOGY) , I did not pay much attention to this....infact I skipped it!.....because it was not clear to me....and I did not understand it,but now I do....atleast to the extent of answering it in the exam if I see it! Please all boses in the house, let us work collaboratively to become more successful in this forthcoming PostUTME. Nobody knows it all.The ones you know,please be kind enough to teach us by posting here and answering questions.... If you have the aforementioned textbook,you can turn to page 178 of chapter 8 and read it explained. It reads in part : Chemical processes of cellular respiration. Cellular respiration occures in two main stages involving a series of chemical reactions and respiratory enzymes. First Stage: Glycolysis ( sugar-breaking process) in the cytoplasm (Anaerobic) The glucose molecule is phosphorylated by an addition of phosphate group to the glucose to become glucose phosphate. Through series of oxidative enzymes , the glucose phosphate is converted to two molecules of triose sugar (a 3-carbon sugar). The triose sugar is converted to pyruvic acid by the removal of four atoms of hydrogen by a co-enzyme called NAD(Nicotinamide adenine dinucleotide) with the formation of 2ATP.
Second Stage:
[b] Anaerobic respiration in Mitochondria If sufficient oxygen is available, each molecule of pyruvic acid(pyruvate) is oxidized to remove one molecule of carbon(IV)oxide(decarboxylation) and two atoms of hydrogen(dehydrogenation) forming one molecule of acetic acid, a 2-carbon acid.Carbon(IV)oxide is released. Krebs' cycle or citric acid cycle The acetic acid enters into a Krebs' cycle where it is joined to a 4-carbon acid(oxaloacetic acid) present in the mitochondrion to form citric acid(a 6-carbon acid) Conclusively, the enzymes of the glycolytic pathway are located in the cytoplasm and the enzymes of the Krebs cycle are located in the mitochondrion. 2 Likes |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:59am On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:59am On Jul 21, 2018 |
Aceed:
A piece of copper ball of mass 20g at 200c
is placed in a copper calorimeter of mass
60g containing 50g of water at 30c,
ignoring heat losses, calculate the final
steady temperature of the mixture.
(shc of water = 4.2JgK)
Note: Specific heat capacity of copper = 0.4 J.g -1K -1 . Solution: Heat lost by copper ball = Heat gained by water + Heat gained by copper calorimeter. M(mass of copper ball) x c(specific heat capacity of copper) x theta(initial temperature of copper) = m(mass of water) x c(specific heat capacity of water) x theta (change in temperature of water after the copper ball has been placed in it) + m(mass of copper calorimeter) x c(specific heat capacity of copper calorimeter) x theta (change in temperature of the calorimeter after the copper ball has been placed in it) .... But we are required to find the final steady temperature...So, Let the final steady temperature be T. Substututing T in the formula above, we have; m x c x theta = m x c x (T- initial temperature of water before the copper ball was placed in it) + m x c x (T- initial temperature of calorimeter before the copper ball was placed in it) Now,substituting the numerical values,we have: 20 x 0.4 x 200 = 50 x 4.2 x (T-30) + 60 x 0.4 x (T-30) 1600 = 210T - 6300 + 24T - 720 1600 = 234T - 7020 1600 + 7020 = 234T 8620 = 234T .: T = 8620/234 = 38.84K (approximately) |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:16am On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:16am On Jul 21, 2018 |
Eben331:
Bro, I can't see the corrections to your biology and chemistry questions on my phone. Try to use signs to represent the correct answers or write it in capital letter. I hope you can now see the corrections clearly? |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:12am On Jul 21, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 12:12am On Jul 21, 2018 |
Correct answers are marked with * DrBESTJC:
Quick Revision Questions in CHEMISTRY
Answer all 25 questions in 20 minutes !
Test of Speed and Accuracy
Typical UI PostUTME questions
Exam is already here !
1) The filter in a cigarette reduces the nicotine content by... ?
A) adsorption *
B) absorption
C) burning
D) evaporation.
2) What mass of K2CrO4 is required to prepare 250cm^3 of 0.020 mol per dm^3 solution...?
A) 97.10g
B) 19.42g
C) 9.70g
D) 0.97g *
[K2CrO4 = 194.2g per mol]
3) The type of reaction that is peculiar to Benzene is...?
A) hydrolysis
B) addition
C) substitution *
D) polymerization
4) Carbohydrates are compounds containing carbon, hydrogen and oxygen in the ratio of...?
A) 1 : 1 : 1
B) 1 : 2 : 1 *
C) 2 : 1 : 1
D) 3 : 1 : 1
5) How many isomers does pentane have...?
A) 4
B) 3 *
C) 5
D) 6
6) What volume of gas is evolved at s.t.p. if 2g of calcium trioxocarbonate (IV) is added to a solution of hydrochloric acid...?
A) 112 cm3
B) 224 cm3
C) 448 cm3 *
D) 2240 cm3
[ Ca = 40, C = 12, O = 16, CL = 35.5, H = 1, Molar volume of a gas at s.t.p. = 22.4 dm3 ]
7) According to Charles's law, the volume of a gas becomes zero at...?
A) 0 degrees centigrade
B) -100 degrees centigrade
C) -273 degrees centigrade *
D) -373 degrees centigrade
8 ) A given volume of methane diffuses in 20seconds. How long will it take the same volume of sulphur(IV)oxide to diffuse under the same conditions...?
A) 5 seconds
B) 20 seconds
C) 40 seconds *
D) 60 seconds
[C = 12, H = 1, S = 32, O = 16]
9) Chlorine consisting of two isotopes of mass numbers 35 and 37 in the ratio of 3:1 has an atomic mass of 35.5. Calculate the relative abundance of the isotope of mass number 37.
A) 20
B) 25 *
C) 60
D) 75
10) The oxidation state of chlorine in HCLO4 is...?
A) -5
B) -1
C) 1
D) 7 *
11) Which of these compounds is a normal salt...?
A) NaHS
B) NaHSO4
C) NaHCO3
D) Na2CO3 *
12) Which of the following acts as both a reducing and an oxidizing agent...?
A) H2
B) SO2 *
C) H2S
D) CO2
13) In the electrolysis of brine, the anode is...?
A) platinum
B) copper
C) zinc
D) carbon *
14) The solubility in mol per dm3 of 20g of CuSO4 dissolved in 100g of water at 180 degrees Centigrade is...?
A) 0.13
B) 0.25
C) 1.25 *
D) 2.00
15) The mass of silver deposited when a current of 10A is passed through a solution of silver salt for 4830 seconds is...?
A) 108.0g
B) 54.0g *
C) 27.0g
D) 13.5g
[Ag = 108, F = 96500 C mol-1 ]
16) The allotrope of carbon used in the decolourization of sugar is ...?
A) graphite
B) soot
C) charcoal *
D) Lampblack
17) Sulphur (IV) oxide bleaches by...?
A) reduction *
B) oxidation
C) hydration
D) adsorption.
18 ) Vulcanization involves the removal of ...?
A) a monomer
B) the single bond
C) the double bond *
D) a polymer
19) CH3COOH (g) ----> CH4(g) + CO2(g)
The reaction above is...?
A) Carboxylation
B) decarboxylation *
C) acidification
D) esterification
20) Alkanol + Alkanoic acid <---(gives reversibly)---> Ester + Water.
The reverse reaction of the equation above is known as...?
A) hydrolysis *
B) saponification
C) hydration
D) fermentation.
21) In the production of soap,concentrated sodium chloride solution is added to...?
A) increase the solubility of the soap
B) decrease the solubility of the soap *
C) saponify the soap
D) emulsify the soap.
22) The property used in the industrial preparation of nitrogen and oxygen from air is...?
A) rate of diffusion
B) solubility
C) density
D) boiling point *
23) A dense white fume is formed when ammonia reacts with...?
A) Hydrogen gas
B) Oxygen gas
C) Hydrogen Chloride gas *
D) Chlorine gas
24) Aluminium hydroxide is used in the dying industry as a...?
A) salt
B) dye
C) mordant *
D) dispersant.
25) The most suitable metal that can be used as a lightning conductor is...?
A) aluminium
B) iron
C) copper *
D) silver. |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:58pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:58pm On Jul 20, 2018 |
The correct answers are marked with * DrBESTJC:
Quick Revision Questions In BIOLOGY
Answer all 25 questions in 20 minutes !
Test of Speed and Accuracy
Typical UI PostUTME Questions
1) The cell of an onion bulb can be differentiated from a cheek cell by the presence of:
A) Plasmalemma
B) Chloroplast
C) Cell wall *
D) Nucleus
2) The membrane surrounding the vacuole in a plant cell is called the:
A) Plasmalemma
B) Tonoplast *
C) Nuclear Membrane
D) Endoplasmic reticulum
3) The pioneer organisms in ecological succession are usually the :
A) Lichens *
B) Algae
C) Ferns
D) Mosses
4) The umbrella-shaped fruiting body of a fully developed muchroom is the :
A) Mycelium
B) Basidium
C) Pileus *
D) Stipe
5) The Cnidoblast cells found in Hydra are used for :
A) Reproduction
B) Offence and Defence *
C) Locomotion and Nutrition
D) Food collection
6) In Spirogyra, the Pyrenoid :
A) Excretes wastes products
B) is suspended to cytoplasmic strands
C) is mainly used for respiration
D) makes the plant skinny to touch
E) usually contains starch *
7) Amoeba moves by means of :
A) Cilia
B) Flagella
C) Pseudopodia *
D) swimmerets
8 ) Which one of the following animals is NEVER secondary host of tapeworms ?
A) Cow
B) Fish
C) Pig
D) Man *
E) Bat
9) The mosquito that transmits elephantiasis in humans is the :
A) Male Culex
B) Female aedes
C) Male aedes
D) Female Culex *
10) In tilapia, the fins lying just behind the gill covers are :
A) Caudal
B) Pectoral *
C) Pelvic
D) Anal
11) The mammalian organ through which nourishment and oxygen diffuses into a developing embryo is called :
A) Amnion
B) Chorion
C) Umbilical cord
D) Oviduct
E) Placenta *
12) The mammalian vein which starts with and ends in a capillary network is the :
A) Pulmonary Vein
B) Mesenteric Vein
C) Renal Vein
D) Hepatic Portal Vein *
13) The mode of nutrition in which digestion is extracellular is :
A) Holophytic
B) Parasitic
C) Holozoic
D) Saprophytic *
14) Insectivorous plants trap and kill their prey to derive :
A) Phosphorus
B) Calcium
C) Nitrogen *
D) Zinc
15) A young plant showing yellowing of leaves is likely to be deficient in :
A) Calcium
B) Magnesium *
C) Potassium
D) Boron
E) Molybdenum
16) Which vitamin plays an important role in blood clotting ?
A) Vitamin A
B) Vitamin K *
C) Vitamin B
D) Vitamin C
17) The part of the stomach nearer the gullet is called the :
A) Epiglottis
B) Cardiac Sphincter *
C) Duodenum
D) Pyloric Sphincter
18) The digestive enzyme that coagulates proteins in milk is :
A) Ptyalin
B) Pepsin
C) Renin *
D) Trypsin
E) Amylase
19) The mammalian heart chamber that pumps blood through the longest distance is the :
A) Right Auricle
B) Right Ventricle
C) Left Ventricle *
D) Left Auricle
20) Excess water in plants is excreted as water vapour and as droplets respectively through :
A) Respiration and Guttation
B) Transpiration and Guttation *
C) Photosynthesis and Guttation
D) Guttation and condensation.
21) An organism with double circulation is the :
A) Duck
B) Rat *
C) Toad
D) Lizard
22) The glottis is the opening which leads to the :
A) Oesophagus
B) Larynx *
C) Nostrils
D) Pharynx
E) Mouth
23) Lung books are used for respiration in :
A) Spiders *
B) Insects
C) Millipedes
D) Snails
24) The gas produced during tissue respiration can be identified by using :
A) Calcium Hydroxide *
B) Calcium Carbonate
C) Copper Sulphate
D) Sodium Hydroxide
25) Fatigue of leg muscles may occur after riding many kilometers on a bicycle because of :
A) Insufficient Glucose
B) Excess carbondioxide
C) Excess Protein
D) Insufficient oxygen * . |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:45pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:45pm On Jul 20, 2018 |
Eben331:
What background colour did you use? White |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:42pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:42pm On Jul 20, 2018 |
Eben331:
Bro, I can't see the corrections to your biology and chemistry questions on my phone. Try to use signs to represent the correct answers. Chai......this one na double wahala ooooo...... Anyway,I will try.....just to make sure that everybody is carried along(and into UI this year.....  ...LOL ! ) |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:08pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:08pm On Jul 20, 2018 |
Aceed:
An electric heater raises the temperature
of 120g of water in a thin light vessel
through 10K in 2min. When placed in 70g
of water contained in a metal vessel of
mass 0.55kg the temperature rises through
9K in the same time. Calculate from the above:
(i) the heat supplied in 2 min by the heater
(ii) the power of the heater
(iii) the heat supplied to the 70g of water
(iv) the heat supplied to the metal vessel
(v) the heat capacity of the vessel
(vi) the specific heat capacity of its material.
(specific heat capacity of water = 4200J.kg-1K-1 )
QUESTION MODIFIED..
Solution: i) Heat supplied by heater = Heat gained by water = m * c*theta = 0.12Kg * 4200 * 10 = 5040Joules in 2 minutes. ii) Power = Energy/time = 5040J/(2 * 60seconds) = 5040J/120seconds = 42Watts. (iii) Heat supplied to the 70g of water = m * c * theta = 0.07Kg * 4200 * 9K = 2646J (iv) Heat supplied by heater = Heat gained by 70g of water + Heat supplied to the metal vessel. But,from our calculations above, Heat supplied by heater = 5040J ......and Heat supplied to the 70g of water = 2646J .: substituting the values,we have that : 5040J = 2646J + Heat supplied to the metal vessel. 5040J - 2646J = Heat supplied to the metal vessel. .: Heat supplied to the metal vessel = 2394J. (v) Heat gained by metal vessel = Heat capacity of the vessel * theta. 2394J = Heat capacity of the vessel * 9K .: Heat capacity of the vessel = 2394/9 = 266(Joule per Kelvin) (vi) Using the formula: Heat Capacity = mass * specific heat capacity .: Specific heat capacity = Heat Capacity/mass = 266/0.55 = 484(Joules per Kilogram per Kelvin) |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:59pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:59pm On Jul 20, 2018 |
Aceed:
A piece of copper of mass 300g at a
temperature of 950c is quickly
transferred to a vessel of negligible
thermal capacity containing 250g of water
at 25c. If the final steady temperature
of the mixture is 100c, calculate the mass
of water that will boil away. (shc of
copper = 400JkgK, shc of water = 4200JkgK,
slh of vapourization of steam = 2260000Jkg)
Solution: Heat lost by copper = Heat gained by water. The explanation of this question is that the hot piece of copper caused the water in a vessel of negligible thermal capacity to boil and some part of it to evaporate. The mass of the water that evaporated is what we are required to find. From the formula above, M(mass of copper) * c(specific heat capacity of copper) * theta(change in temperature of the piece of copper when it was transfered into the water vessel) = m(mass of water) * c(specific heat capacity of water) * theta(change in temperature of water when the piece of copper was inserted into it) + m(mass of water that will boil away) * L v (Latent heat of vaporization of steam). That is: M*c*theta = m*c*theta + m*L vConvert 300g and 250g respectively to Kg by dividing by 1000 to get 0.3Kg and 0.25g. Substituting the values in the formula above; 0.3 * 400 * (950-100) = 0.25 * 4200 * (100-25) + 2260000m 102000 = 78750 + 2260000m 102000 - 78750 = 2260000m 23250 = 2260000m m = 23250/2260000 = 0.01029Kg = 10.29g (approximately) |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:58pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:58pm On Jul 20, 2018 |
BiafranDel:
DRbestjc
1. B
2. D
3. C
4. B
5. A
6. C
7. B
8. A
9. B
1O. D
11. D
12. B
13. D
14. C
15. B
16. C
17. A
18. B
19. B
2O. A
21. B
22. D
23. D
24. C
25. C
FOR THE NUMBER 6. The answer i got was not included. 0.448. So i just choose C being closest. Post the correct answers for proper clarification. The answer you got is still in dm^3....but the answer given is in cm^3. |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:46pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:46pm On Jul 20, 2018 |
Corrections. Correct answers in bold DrBESTJC:
Quick Revision Questions in CHEMISTRY
Answer all 25 questions in 20 minutes !
Test of Speed and Accuracy
Typical UI PostUTME questions
Exam is already here !
1) The filter in a cigarette reduces the nicotine content by... ?
A) adsorption
B) absorption
C) burning
D) evaporation.
2) What mass of K2CrO4 is required to prepare 250cm^3 of 0.020 mol per dm^3 solution...?
A) 97.10g
B) 19.42g
C) 9.70g
D) 0.97g
[K2CrO4 = 194.2g per mol]
3) The type of reaction that is peculiar to Benzene is...?
A) hydrolysis
B) addition
C) substitution
D) polymerization
4) Carbohydrates are compounds containing carbon, hydrogen and oxygen in the ratio of...?
A) 1 : 1 : 1
B) 1 : 2 : 1
C) 2 : 1 : 1
D) 3 : 1 : 1
5) How many isomers does pentane have...?
A) 4
B) 3
C) 5
D) 6
6) What volume of gas is evolved at s.t.p. if 2g of calcium trioxocarbonate (IV) is added to a solution of hydrochloric acid...?
A) 112 cm3
B) 224 cm3
C) 448 cm3
D) 2240 cm3
[ Ca = 40, C = 12, O = 16, CL = 35.5, H = 1, Molar volume of a gas at s.t.p. = 22.4 dm3 ]
7) According to Charles's law, the volume of a gas becomes zero at...?
A) 0 degrees centigrade
B) -100 degrees centigrade
C) -273 degrees centigrade
D) -373 degrees centigrade
8 ) A given volume of methane diffuses in 20seconds. How long will it take the same volume of sulphur(IV)oxide to diffuse under the same conditions...?
A) 5 seconds
B) 20 seconds
C) 40 seconds
D) 60 seconds
[C = 12, H = 1, S = 32, O = 16]
9) Chlorine consisting of two isotopes of mass numbers 35 and 37 in the ratio of 3:1 has an atomic mass of 35.5. Calculate the relative abundance of the isotope of mass number 37.
A) 20
B) 25
C) 60
D) 75
10) The oxidation state of chlorine in HCLO4 is...?
A) -5
B) -1
C) 1
D) 7
11) Which of these compounds is a normal salt...?
A) NaHS
B) NaHSO4
C) NaHCO3
D) Na2CO3
12) Which of the following acts as both a reducing and an oxidizing agent...?
A) H2
B) SO2
C) H2S
D) CO2
13) In the electrolysis of brine, the anode is...?
A) platinum
B) copper
C) zinc
D) carbon
14) The solubility in mol per dm3 of 20g of CuSO4 dissolved in 100g of water at 180 degrees Centigrade is...?
A) 0.13
B) 0.25
C) 1.25
D) 2.00
15) The mass of silver deposited when a current of 10A is passed through a solution of silver salt for 4830 seconds is...?
A) 108.0g
B) 54.0g
C) 27.0g
D) 13.5g
[Ag = 108, F = 96500 C mol-1 ]
16) The allotrope of carbon used in the decolourization of sugar is ...?
A) graphite
B) soot
C) charcoal
D) Lampblack
17) Sulphur (IV) oxide bleaches by...?
A) reduction
B) oxidation
C) hydration
D) adsorption.
18 ) Vulcanization involves the removal of ...?
A) a monomer
B) the single bond
C) the double bond
D) a polymer
19) CH3COOH (g) ----> CH4(g) CO2(g)
The reaction above is...?
A) Carboxylation
B) decarboxylation
C) acidification
D) esterification
20) Alkanol Alkanoic acid <---(gives reversibly)---> Ester Water.
The reverse reaction of the equation above is known as...?
A) hydrolysis
B) saponification
C) hydration
D) fermentation.
21) In the production of soap,concentrated sodium chloride solution is added to...?
A) increase the solubility of the soap
B) decrease the solubility of the soap
C) saponify the soap
D) emulsify the soap.
22) The property used in the industrial preparation of nitrogen and oxygen from air is...?
A) rate of diffusion
B) solubility
C) density
D) boiling point
23) A dense white fume is formed when ammonia reacts with...?
A) Hydrogen gas
B) Oxygen gas
C) Hydrogen Chloride gas
D) Chlorine gas
24) Aluminium hydroxide is used in the dying industry as a...?
A) salt
B) dye
C) mordant
D) dispersant.
25) The most suitable metal that can be used as a lightning conductor is...?
A) aluminium
B) iron
C) copper
D) silver. |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:55pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:55pm On Jul 20, 2018 |
BiafranDel:
answer is c. glycolysis occurs in the cytoplasm while kreb's cycle occurs in the mitochondria directly Please, do you have any reference to this..??....as in the textbook where you saw it? |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:49pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:49pm On Jul 20, 2018 |
esivue007:
im stil thinking and googling hard 4 d question cuz i think its mitochondria..... Yes....google said it's mitochondria....but 'BiafranDel' said it is cytoplasm....I'm confused myself....... how can almighty google be wrong ? ........just thinking..... |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:44pm On Jul 20, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 4:44pm On Jul 20, 2018 |
I have successfully completed my registration today.......it was easier than I thought... |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:06am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 11:06am On Jul 19, 2018 |
Eben331:
(1) A swimmer is capable of swimming at 1.4m/s in still water. (a) how far downstream will be land if he swims directly across a 180m wide river? (b) how long will it take him to reach the other side? Abeg Big Boses in the house,...Help me with this question...I don solve am tire.... |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:37am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:37am On Jul 19, 2018 |
BiafranDel:
yeah. I am right. Cytoplasm is the correct answer. Alright......Noted. |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:11am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 10:11am On Jul 19, 2018 |
Aceed:
Which of the following compounds is
not obtained by polymerization?
(a) Plastic
(b) Polythene
(c) Petroleum
(d) Cellulose
Answer: C(Petroleum) Reason: 1) Plastics Plastics are polymers whose molecules can slide over one another when stretched, with the molecules remaining in their new positions when the stretching force is removed, i.e. for the material to become deformed. Plastics of all kinds are synthetic polymers. They are light, soft or hard, flexible,tough and with great tensile strenghts. They are water proof, resistant to chemicals and heat. Plastics are non-biodegradable ; hence, plastic wastes usually accumulate in the soil and pollute the environment. 2) Types of Plastics A) Thermoplastics Thermoplastics are polymers that can be softened or melted when subjected to heat or pressure, and can be remoulded into any shape. For such polymers, their plasticity (plastic property) increases as the heat or pressure applied increases. Polyethene is an example of a thermoplastic. B) Thermostats Thermostats are polymers that cannot be softened or melted by heat or pressure. Once formed,they become set(rigid) and cannot be remoulded again. An example is polychloroethene(PVC). 3) Petroleum Petroleum or crude oil occurs as a dark brown viscous liquid with unpleasant smell. It must have been formed millions of years ago by the decay and decomposition of marine organisms(plants and animals) after being subjected to high temperature and pressures in the abscence of air, and then trapped in porous rocks deep down the earth's crust, together with natural gas. 4) Cellulose Polysaccharides are polymerisation products of thousands of molecules of monosaccharides(the hexoses). They include: - Starch - Cellulose - Glycogen(animal starch) - Inulin. Cotton fibers are the purest form of cellulose.Nearly 90% of the cotton fibers are cellulose,making it nature's most abundant polymer. From the above discussions,it is obvious that petroleum is not a product of polymerisation |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:02am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 9:02am On Jul 19, 2018 |
BiafranDel:
answer is c. glycolysis occurs in the cytoplasm while kreb's cycle occurs in the mitochondria directly You may be right...I am not so sure of that question,....that was why I refered him to google. Please sir,we also need your contributions here in solving problems. Let's teach ourselves and perform better...! |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:14am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 8:14am On Jul 19, 2018 |
Aceed:
Which of the following is not a
component of guard cells?
(a) Chloroplasts
(b) Nucleus
(c) thin inner wall
(d) rough spike
Answer: D Reason:
See the picture below 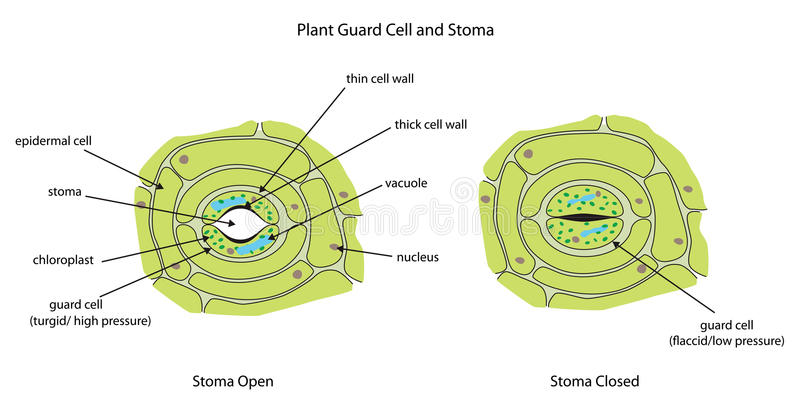
|
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:38am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:38am On Jul 19, 2018 |
Aceed:
The enzyme of the glycolytic pathway
is located in the?
(a)mitochondria
(b) golgi apparatus
(c) cytoplasm
(d) Nucleus
Answer: A Reason Glycolytic Enzymes are Located on the outside of the Mitochondrion. Google can give more details on this... |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:13am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 7:13am On Jul 19, 2018 |
Aceed:
The products of photochlorination of
ethanoic acid are?
Answer Substitution Reactions in Alkanoic Acid.
Halogenation Reaction When chlorine or bromine gas is passed into a mixture of an alkanoic acid (except methanoic acid) and a small amount of phosphorus in the presence of sunlight, 2-chloroalkanoic acid or 2- bromoalkanoic acid is formed. The photochlorination of ethanoic acid produces 2-chloroethanoic acid and Hydrogen Chloride gas.
Equation of the reaction is: CH 3COOH + CL 2 ----> CLCH 2COOH + HCL Note: Substitution occures on the carbon directly attached to the carboxyl carbon (i.e. The alpha-carbon atom ) . |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 6:15am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 6:15am On Jul 19, 2018 |
Aceed:
What are the products of the reaction
between ethyl ethanoate and ammonia?
Answer When ammonia is bubbled through an alkanoate(ester), the corresponding amide and alkanol are produced. This is known as ammonalysis. Ammonia reacts with ethylethanoate to produce a mixture of ethanamide and ethanol.
Equation of the reaction: CH 3COOCH 2CH 3 + NH 3 ------> CH 3CONH 2 + CH 3CH 2OH |
 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:34am On Jul 19, 2018 Education / Re: UI 2018/2019 Admission Thread by DrBESTJC(m): 5:34am On Jul 19, 2018 |
Aceed:
Effervescence occurs when water comes
in contact with
(a) calcium
(b) copper
(c) sulphur
(d) lead
Answer: A (Calcium) Reason Potassium,sodium or calcium metal reacts with cold water to liberate hydrogen gas and giving an alkaline solution. However, the reaction with potassium or sodium is very violent. Reaction of cold water with calcium metal When a small piece of calcium metal is placed in a beaker of cold water,it sinks. A boiling tube full of water is then inverted over it and the metal dissolves with an effervescence or evolution of an odourless and colourless gas which is hydrogen. Hydrogen can be collected by upward delivery or downward displacement of air;since it is less dense than air being the lightest known gas. As the calcium dissolves, a white suspension of calcium hydroxide, which is slightly soluble in water is formed. Hence, if the water in the beaker is initially coloured with a few drops of methyl orange , the solution changes from orange to yellow ....and if phenolphthalein is used, the colour change is from colourless to pink. This is because the resulting solution is alkaline. Equation of the reaction. Ca(s) + 2H 2 O(L) ----> Ca(OH) 2 + H 2 |



 ...LOL ! )
...LOL ! )